how many electrons do halogens have|6.12: Halogens : Clark How many valence electrons do halogens have? Halogens have 7 valence electrons. This makes them very reactive as they seek to complete their outermost shell of 8 electrons. 3. Where are the halogens . Sarinah Thamrin Plaza, Jakarta : Lihat ulasan, artikel, dan foto Sarinah Thamrin Plaza di antara objek wisata di Jakarta di Tripadvisor. Lompat ke konten utama. Telusuri. Trip. Ulasan. IDR. . Selain itu karena letaknya dekat pusat jantung Jakarta ( hotel Indonesia) anda juga akan mudah mengakses MRT Jakarta dan Trans Jakarta yang bisa .
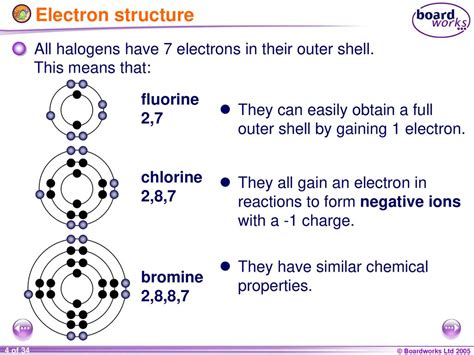
how many electrons do halogens have,Summary. The halogens all have seven electrons in their outer shells. The electron configuration in the outer shell is \ (ns^2np^5\). As the atomic number increases, the reactivity of the halogens decreases. . Because the halogen elements have seven valence electrons, they only require one additional electron to form a full octet. This characteristic makes them more .The halogens fluorine, chlorine, bromine, and iodine are nonmetals; the chemical properties of the two heaviest group 17 members have not been conclusively investigated. The halogens show trends in chemical bond energy moving from top to bottom of the periodic table column with fluorine deviating slightly. It follows a trend in having the highest bond energy in compounds wit. How many valence electrons do halogens have? Halogens have 7 valence electrons. This makes them very reactive as they seek to complete their outermost shell of 8 electrons. 3. Where are the halogens .
Because the halogen elements have seven valence electrons, they only require one additional electron to form a full octet. This characteristic makes them more reactive than .
Each halogen has seven valence electrons. The outermost electronic configuration of halogen is represented by the general formula ns 2 np5. All elements, with the last electron being added to one of the orbitals of their . These reactive nonmetals have seven valence electrons. As a group, halogens exhibit highly variable physical properties. Halogens range from solid (I 2) to liquid (Br 2) to gaseous (F 2 and Cl 2) at room .Electronic Configuration- Halogen Group. The group 17 elements of the periodic table are made up of five chemically similar elements: Fluorine. Chlorine. Bromine. Iodine. Astatine. The term halogen has been derived .
Atoms of belonging to the halogen group have 7 electrons in their outermost (valence) shell. These atoms need one more electron in order to have a stable octet. The usual oxidation state of a halogen atom .
The ions have a stable arrangement of electrons, with a complete outer shell. Reactions with metals The halogens react with metals to produce salts close salt The substance formed when the .

The Halogen family elements have how many valence electrons? Halogen family members, or elements in group 17, have 7 valence electrons.Flexi Says: The halogens also form single covalent bonds in their diatomic molecules. An atom of any halogen, such as fluorine, has seven valence electrons. Its unpaired electron is located in the 2 p orbital. Discuss further with Flexi. Ask your own question! The halogens also form single covalent bonds in their diatomic molecules. An . List of Halogens. Depending on who you ask, there are either 5 or 6 halogens. Fluorine, chlorine, bromine, iodine, and astatine definitely are halogens. Element 117, tennessine, might have some properties in common with the other elements. Even though it is in the same column or group of the periodic table with the other halogens, .how many electrons do halogens have 6.12: Halogens The halogens all have seven electrons in their outer shells. The electron configuration in the outer shell is \(ns^2np^5\). As the atomic number increases, the reactivity of the halogens decreases. Fluorine and chlorine exist as gases at room temperature, while bromine is a liquid, and iodine is a solid.Table 1.6: Electron Affinity of Halogens; Halogen Electron Affinity (kJ/mol) Fluorine-328.0: Chlorine-349.0: Bromine-324.6: Iodine-295.2: Astatine . As a general rule, halogens usually have an oxidation state of -1. However, if the halogen is bonded to oxygen or to another halogen, it can adopt different states: the -2 rule for oxygen takes . Summary. The halogens all have seven electrons in their outer shells. The electron configuration in the outer shell is ns2np5 n s 2 n p 5. As the atomic number increases, the reactivity of the halogens decreases. Fluorine and chlorine exist as gases at room temperature, while bromine is a liquid, and iodine is a solid.Halogens: The halogens are the elements present in group 7 of the periodic table. A group is referred to as a column of elements. These elements are fluorine (F), chlorine (Cl), bromine (Br), Iodine (I), astatine (At), and Tennessine (Ts). They are grouped together as they have similar chemical reactivities because they have the same number of . The halogens all have the general electron configuration ns 2 np 5 , giving them seven valence electrons. Do all halogens have valence electrons? The halogens are among the most reactive of all elements, although reactivity declines from the top to the bottom of the halogen group. Because all halogens have seven valence electrons, .

Every subshell has a # of orbits s/p/d/f that can each hold 2 electrons each (one has the opposite spin of the other). The first shell (of all atoms) has 1 subshell of s-orbitals containing 1 s orbital. This means that the first shell can hold 2 electrons. The second shell has 2 subshells: 1 s-orbital and 3 p-orbitals.This page discusses the trends in the atomic and physical properties of the Group 7 elements (the halogens): fluorine, chlorine, bromine and iodine. Sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility, including a discussion of the bond enthalpies of halogen-halogen . Valence electrons: For main group elements (i.e s-block and p-block elements), the valence electrons are the electrons present in the outermost orbit. But for most of the transition and inner transition . the halogens tend to gain electrons. lose. Halogens have 7 valence electrons, in order to become a noble gas you need 8 so instead of losing electrons, it would be easier just to gain 1. That . Halogen Properties. The halogens share several common properties: All of the halogens are nonmetals. They are poor conductors of heat and electricity and form brittle solids. Atoms of halogen elements have seven valence electrons in their outer shell. This is one less electron than needed for a full valence shell, so their usual oxidation .
Question: How many valence electrons do the halogens possess? Group of answer choices 7 1 6 5 2. How many valence electrons do the halogens possess? There are 2 steps to solve this one.how many electrons do halogens haveGeneral Properties of the Alkali Metals. Various properties of the group 1 elements are summarized in Table 20.4.1. In keeping with overall periodic trends, the atomic and ionic radii increase smoothly from Li to Cs, and the first ionization energies decrease as . Properties of the Halogens . These reactive nonmetals have seven valence electrons. As a group, halogens exhibit highly variable physical properties. Halogens range from solid (I 2) to liquid (Br 2) to gaseous (F 2 and Cl 2) at room temperature. As pure elements, they form diatomic molecules with atoms joined by nonpolar covalent bonds.
sulfur. helium. potassium. aluminum. Solution. Sulfur (S) is located in Group VIA (Group 16), so it has 6 valence electrons. Helium (He) is located in Group VIIIA (Group 18). However, one atom only has two electrons, so it could never have more than 2 valence electrons. As noted above, helium is the only exception for the main group .
how many electrons do halogens have|6.12: Halogens
PH0 · List of Halogens (Element Groups)
PH1 · Halogens: General Characteristics & Physical properties
PH2 · Halogen Elements and Properties
PH3 · Halogen
PH4 · Group 17: The Halogens
PH5 · Group 17: General Properties of Halogens
PH6 · Electronic Configuration
PH7 · 8.13: The Halogens
PH8 · 6.12: Halogens